Consider the LUMA Study for your patients with treatment-naïve, early-stage Parkinson’s disease
Evaluating whether early intervention may potentially slow disease progression
About the LUMA Study
The LUMA Study is a Phase 2b, randomized, double-blind, placebo-controlled study to determine the efficacy and safety of an oral study drug, BIIB122, in patients with early-stage Parkinson’s disease (PD).
Targeting patients earlier in their disease may have greater effect on PD symptoms and progression. In early-stage disease, when there is less neurodegeneration in the dopaminergic neurons, the potential exists to restore or preserve function in a higher percentage of neurons than in later-stage disease.1
About the Study Medication2-9
BIIB122, an LRRK2 inhibitor, is a potential approach for treating PD patients. Increased LRRK2 activity disrupts normal lysosomal function, and abnormal lysosomal function plays a central role in the pathology of PD. Increased LRRK2 activity is observed in patients with and without LRRK2 mutations. LRRK2 inhibition improves lysosomal function and protein processing, and may slow the progression of PD.
About the LUMA Study
Targeting patients earlier in their disease may have greater effect on PD symptoms and progression. In early-stage disease, when there is less neurodegeneration in the dopaminergic neurons, the potential exists to restore or preserve function in a higher percentage of neurons than in later-stage disease.1

About the Study Medication2-9
BIIB122, an LRRK2 inhibitor, is a potential approach for treating PD patients. Increased LRRK2 activity disrupts normal lysosomal function, and abnormal lysosomal function plays a central role in the pathology of PD. Increased LRRK2 activity is observed in patients with and without LRRK2 mutations. LRRK2 inhibition improves lysosomal function and protein processing, and may slow the progression of PD.
Study Design
- The study duration is approximately 1 to 3 years including up to 26 in-office visits and up to 3 remote
- > The treatment period will last a minimum of 48 weeks to a maximum of 144 weeks
- > There will be a 2-week study follow-up period
- Participants will be randomized to receive BIIB122 or placebo in a 1:1 ratio
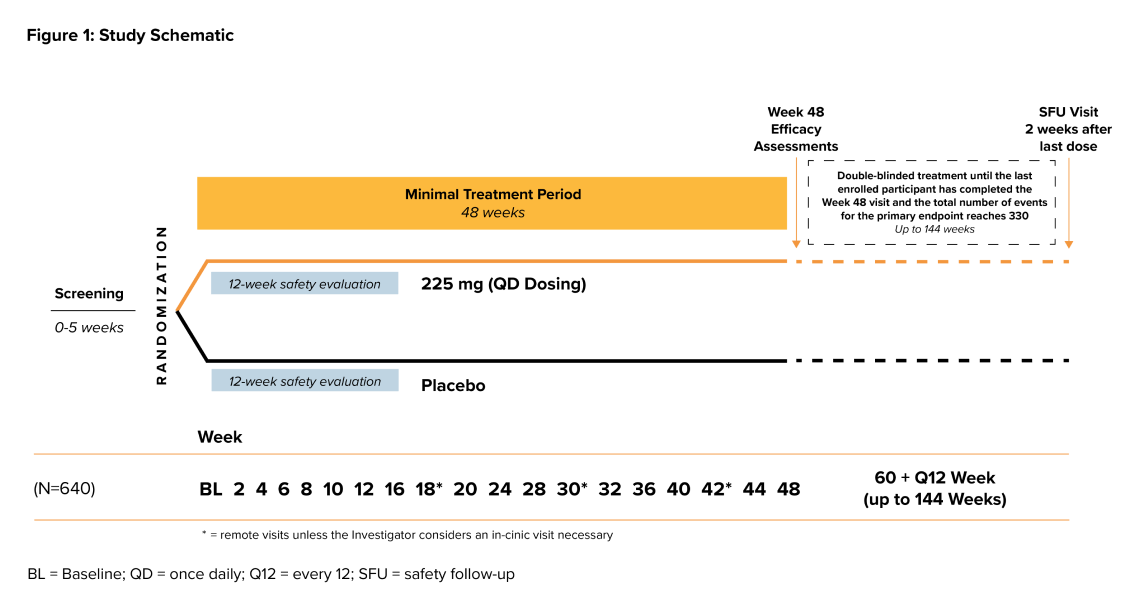
Study Design
- The study duration is approximately 1 to 3 years including up to 26 in-office visits and up to 3 remote
- > The treatment period will last a minimum of 48 weeks to a maximum of 144 weeks
- > There will be a 2-week study follow-up period
- Participants will be randomized to receive BIIB122 or placebo in a 1:1 ratio

Key Eligibility Criteria*
- Aged 30 to 80 years
- Diagnosed with Parkinson’s disease within the last 2 years and at least 30 years of age at time of diagnosis
- Never treated with PD medications (or treated with PD medications for
< 30 days) - Not expected to start PD medications for at least 48 weeks
*Additional eligibility criteria apply.
Patients Who Qualify and Decide to Participate in the Study Will Receive:
- All study-related care and study medication at no charge
- Regular monitoring of symptoms and health by physicians who specialize in Parkinson’s disease
- Reimbursement for transportation and rideshare to attend study visits, as needed
- The opportunity to contribute to learning more about Parkinson’s disease

Key Eligibility Criteria*
- Aged 30 to 80 years
- Diagnosed with Parkinson’s disease within the last 2 years and at least 30 years of age at time of diagnosis
- Never treated with PD medications (or treated with PD medications for
< 30 days) - Not expected to start PD medications for at least 48 weeks
*Additional eligibility criteria apply.
Patients Who Qualify and Decide to Participate in the Study Will Receive:
- All study-related care and study medication at no charge
- Regular monitoring of symptoms and health by physicians who specialize in Parkinson’s disease
- Reimbursement for transportation and rideshare to attend study visits, as needed
- The opportunity to contribute to learning more about Parkinson’s disease
For more information or to refer a patient
Click the link below to visit ParkinsonsResearchStudies.com
To refer a patient, please click the link below to complete the online referral form
Click the link below to download the patient brochure
For more information or to refer a patient
Click the link below to visit ParkinsonsResearchStudies.com
To refer a patient, please click the link below to complete the online referral form
Click the link below to download the patient brochure
References:
- Cheng H-C, Ulane CM, Burke RE. Clinical progression in Parkinson’s disease and the neurobiology of axons. Ann Neurol. 2010;67(6):715-725. doi:10.1002/ana.21995
- Wallings RL, Humble SW, Ward ME, Wade-Martins R. Lysosomal dysfunction at the centre of Parkinson’s disease and frontotemporal dementia/amyotrophic lateral sclerosis. Trends Neurosci. 2019;42(12):899-912. doi:10.1016/j.tins.2019.10.002
- Dehay B, Martinez-Vicente M, Caldwell GA, et al. Lysosomal impairment in Parkinson’s disease. Mov Disord. 2013;28(6):725-732. doi:10.1002/mds.25462
- DiMaio R, Hoffman EK, Rocha EM, et al. LRRK2 activation in idiopathic Parkinson’s disease. Sci Transl Med. 2018:10(451):eaar5429. doi:10.1126/scitranslmed.aar5429
- Cellular mechanisms of LRRK2 in health and disease. Michael J. Fox Foundation. Accessed August 7, 2023. https://www.michaeljfox.org/grant/cellular-mechanism-lrrk2-health-and-disease
- Khan NL, Jain S, Lynch JM, et al. Mutations in the gene LRRK2 encoding dardarin (PARK8) cause familial Parkinson’s disease: clinical, pathological, olfactory and functional imaging and genetic data. Brain. 2005;128(Part 12):2786-2796. doi:10.1093/brain/awh667
- Jaleel M, Nichols RJ, Deak M, et al. LRRK2 phosphorylates moesin at threonine-558: characterization of how Parkinson’s disease mutants affect kinase activity. Biochem J. 2007;405(2):307-317. doi:10.1042/BJ20070209
- West AB, Moore DJ, Choi C, et al. Parkinson’s disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Human Mol Gen. 2007;16(2);223-232. doi:10.1093/hmg/ddl471
- Sheng Z, Zhang S, Bustos D, et al. Ser1292 autophosphorylation is an indicator of LRRK2 kinase activity and contributes to the cellular effects of PD mutations. Sci Transl Med. 2012;4(164):164ra161. doi:10.1126/scitranslmed.3004485
